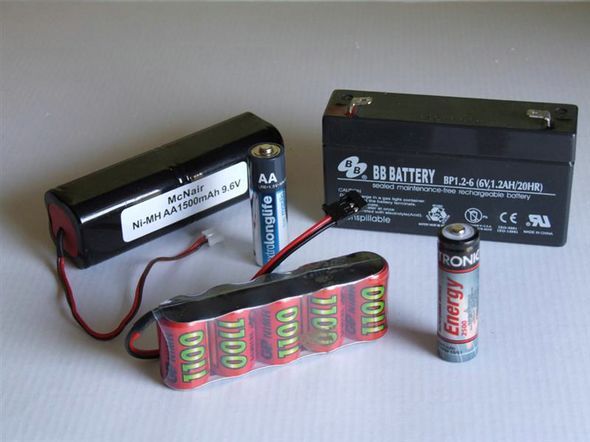
L to R back: 9.6v NiMH transmitter pack, AA alkaline cell, 6v sealed lead acid battery, L to R front: 6v NiMH receiver pack, AA NiMH cell.
Enjoy more Model Boats Magazine reading in the monthly magazine.
Click here to subscribe & save.
Fundamentals
Many types of cells are in use for various applications. Almost any two materials can be used as electrodes with a third substance as the electrolyte. Most of us have seen the digital clock powered by a cell created by sticking two metal rods in a lemon or a potato. Many of these cell types are either prohibitively expensive to make, are unsuitable for our purposes, or are not readily available. So, this article aims to provide general information on the characteristics of the most common types of cell and how these influence the choice of them for different uses, particularly within our hobby.
The main types of cell in general use by model boaters are Lead Acid, NiCd, NiMH and the ubiquitous Alkaline primary cell which is sometimes used for transmitter and receiver power, but rarely for motive use. Photo 1 shows the common cells and batteries in use by model boaters. Lithium Ion secondary cells and batteries (pictured later) tend to be used primarily for electric flight and require charging facilities that the average model boater may not possess. However, before we can usefully discuss the characteristics of different cell types in detail, we need to define a few terms.
Terminology
Throughout this article the term cell rather than battery will be used when referring to a single unit. The term battery will be used when a number of cells are connected together in series and/or parallel, to provide a higher voltage and/or greater capacity.
Cell: A unit having three components which are the two electrodes; a cathode and anode immersed in or separated by a third component, the electrolyte. This reacts with the electrodes to convert chemical energy to electrical energy, Diagram 1.
Primary Cell: One in which the chemical process taking place during discharge is non-reversible. The cell is useless once any one of the components is exhausted.
Secondary cell: One in which the chemical process is reversible during the recharging process. These cells can be reused many times, although the ability to retain charge may deteriorate at a rate depending on the charge/discharge history.
Anode: The electrode where oxidation (loss of electrons) takes place. While discharging, it is the negative electrode; however, during the charging process it becomes the positive electrode.
Cathode: The electrode where reduction (gain of electrons) takes place. While discharging it is the positive electrode; however, during charging it becomes the negative electrode.
Electrolyte: The medium which provides the ion transport mechanism between the positive and negative electrodes of a cell.
Energy density: A measure of energy available from a cell per unit mass or volume. Units are Watt-hours per kilogram (Wh/kg) or litre (Wh/l), Diagram 2.
Ampere-hour (often abbreviated to Ah): A measure of the energy holding capacity of a cell. One ampere-hour is the amount of electric charge transferred by a steady current of one ampere in one hour. Although it is not a direct measure of the energy in a cell, it is a useful indicator of how long a battery will last in a given supply configuration. The commonly seen milli-Ampere hour (mAh) is one thousandth of an Ampere-hour.
C (capacity): Quoted in Ah or mAh, this gives an indication of the total energy contained in the cell. This can theoretically be discharged at any rate. A 1Ah battery can (in theory) discharge at 1 amp for 1 hour (C rate) or at 0.1 amps for 10 hours (C/10 rate) or at 10 amps for 0.1 hour (10C rate). In all cases the total energy supplied is 1Ah. However this theoretical situation is not practically achievable, mainly due to potential cell damage from overheating at high discharge rates. C is mainly used in discussion of recharging rates which are generally quoted in terms of this parameter.
Cycle life: The number of charge /discharge cycles expected from a particular cell chemistry and its construction when in normal use. Cycle life may be reduced by fast charging regimes.
Self-discharge: This occurs when a cell is left unused for long periods. Different cell types have different self-discharge rates.
Internal resistance: The apparent resistance of a cell. This limits the discharge rate as it causes a voltage drop across the cell when current is drawn.
Series connection: Cells in series are connected with the anode of one cell joined to the cathode of the next cell. If n cells of nominal voltage V and capacity C are connected in series, the resulting battery has a nominal voltage of n times voltage V and a capacity of C, Diagram 3.
Parallel connection: Cells in parallel are connected with the anode of one cell joined to the anode of the next. If n cells of nominal voltage V and capacity C are connected in parallel, the resulting battery has a nominal voltage of V volts and a capacity of n times C, Diagram 4.
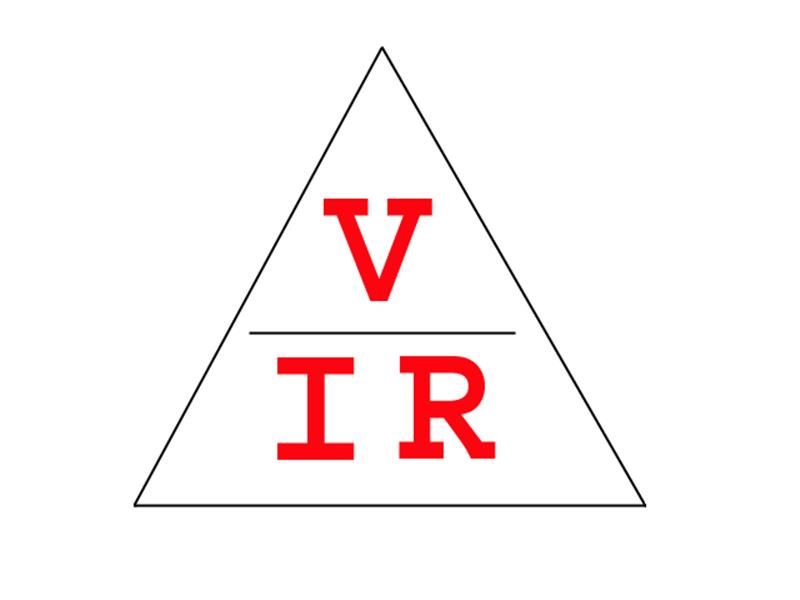
Ohms law: The classic law linking voltage (V), current (I) and resistance (R). Ohms Law allows any one of the parameters to be calculated if the other two are known. Remembering all three possible equations can be difficult, but theres a useful tip I learnt from my physics master some 50 years ago! Remember this little diagram. If you cover up the unknown parameter you have the equation for the other two. Hence V = IR, I = V/R and R = V/I.
With those terms defined, lets have a look at specific cell chemistries. The most common type of primary cell in general use is the aforementioned alkaline cell, which uses Manganese Dioxide and Zinc electrodes with an electrolyte of Potassium Hydroxide. The construction of the much used AA cell involves a Swiss roll style assembly of anode and cathode sheets with electrolyte and provides a large capacity in a small package (an energy density of around 110 Wh/kg). The alkaline cell has an output voltage of 1.5 volts. Its discharge characteristic shows a gradual reduction in voltage as the cell approaches exhaustion, Diagram 5. This is very different from the characteristic of both NiCd and NiMH cells. This can be an advantage in applications which are not particularly voltage sensitive. In these cases erratic operation may be a clue that a battery is reaching exhaustion before the circuit ceases to function, whereas the sharper curve of NiMH or NiCd batteries may give a sudden loss of function.
From time to time advertisements appear offering gadgets claiming to rejuvenate alkaline cells. These are of questionable value and may be dangerous, as alkaline cells are not designed to be recharged and may overheat and explode.
Moving on to secondary cells, lead acid is one of the oldest technologies in general use. It uses electrodes of lead and lead dioxide with dilute sulphuric acid as an electrolyte. Older wet cell types such as car batteries are not suitable for use in boats due to the risk of acid spillage, but modern sealed or gel cell batteries are fine. These cells have a low cycle life, a rapid self discharge and low energy densities (normally between 30 and 40 Wh/kg). However, the comparatively low price makes them attractive in some applications. They can easily be accommodated in larger boats and provide a handy source of ballast. Each cell has an output voltage of two volts when fully charged and they are generally available in 6 volt (three cell) or 12 volt (six cell) batteries. During discharge the cell voltage falls gradually, Diagram 6.
Like a NiCd cell, an NiMH cell maintains a substantially constant voltage as it discharges with a sudden voltage drop as the fully discharged state is neared, Diagram 7.
The capacity of standard NiMH AA cells has been increased considerably since they first became easily available. The first ones I remember buying a few years had a capacity of 700mAh; the ones I bought recently have a capacity of 2700mAh and cost much less.
Chargers for NiMH cells are similar to those used for NiCd cells. The recommended charge rate is C/10 for longest cell cycle life, but faster rates can be used if a suitable charger is available, preferably with overheat monitoring.
Rechargeable Lithium cells come in two main varieties, Lithium Ion and Lithium Polymer (LiPo). Lithium Ion batteries are found mainly in laptop computers and small domestic items like video cameras and they have a rigid case. The energy density is about twice that of an NiMH cell (140Whr/kg) and the nominal cell voltage 3.6 volts. There is evidence that these cells lose capacity due to ageing over two or three years whether in use or not. On the other hand self-discharge is less than half that of nickel based cells.
Lithium Polymer cells are a further development of the Lithium Ion technology. The essential difference is that they do not need a rigid case which frees up the manufacturer to produce cells in a much lighter form with polyester laminate foil cases, Photo 2. These cells, in various battery configurations, are becoming increasingly popular in a number of model applications. The electric flying fraternity were the first to appreciate the benefits of light weight and high energy density. They were also the first to experience the catastrophic effects of mistreating LiPo cells. The cell chemistry is complex, as is the internal structure of the cell. Suffice it to say that the reaction depends on the migration of lithium ions rather than electrons and that the electrodes are complex compounds of lithium and other metals. The reference to polymer in the name of the cell is because the electrolyte in the cell is held in a polymer structure. The nominal cell output voltage is 3.7 volts when fully charged and if it is allowed to drop below 3 volts during discharge this will permanently damage the cells capacity.
When made up into multi-cell batteries they have a unique system of describing the capacity of the battery which identifies the number of cells in the battery and the series and parallel configuration. The identifier consists of the following, (number)S(number)P. The first number indicates the number of cells in series, the second the number of series units then paralleled. To give a couple of examples, 2S0P is a battery with two cells in series giving an output voltage of approximately 7.4 volts. A more complex code might be 3S4P which defines a battery consisting of chains of 3 cells in series (3S), with 4 of these chains then wired in parallel (4P). Such a battery would have a nominal voltage of 11.1 volts and a capacity of 4C where C is the capacity of a single cell, Diagram 8.
With a cycle life of around 500, energy density vastly superior to NiMH cells and the ability to discharge at high multiples of C, Diagram 9, its easy to see why LiPo batteries are seen in some quarters as very close to heaven on earth. However there have been well-publicised incidents involving Lithium ion laptop computer batteries self-combusting and at least one major manufacturer has had to withdraw a large number of such batteries. Foil pouch LiPo batteries are no less susceptible to abuse, both electrical and physical. It is therefore important that great care is taken in the storage, transport, use and recharging of LiPo batteries. Only chargers specifically designed for LiPo batteries must be used with the charge regime carefully matched to the battery. The soft battery pack should be placed in a fire resistant enclosure during the recharge cycle and monitored visually during the process. Some LiPo batteries have extra leads in addition to the main positive and negative connector. These are provided to facilitate differential charging of individual cells which can be undertaken by some of the sophisticated chargers needed to manage LiPo batteries safely. Having taken the decision to use LiPo batteries, another wise move is to use an ESC (Electronic Speed Controller) which has an adjustable cut off voltage which can be set at three volts to prevent discharge beyond this point and consequent damage to the battery.
There are a number of other precautions which need to be taken when using LiPo cells. These are documented on the websites of the well known battery retailers, who no doubt wish to ensure that purchasers are fully aware of the care needed in the use of these batteries. I wont detail them all here as I feel that careful research should be an essential part of the decision to buy into this technology. So summing up, LiPo batteries offer high returns in terms of energy density for propulsion purposes, NiCds have the lowest internal resistance and still have a place in applications where fast discharge rates are required, but NiMH cells seem destined to be the future workhorse technology, particularly for transmitters and receivers for some time to come.
Disposal and recycling
Other useful internet resources
Information on the safe use of LiPo batteries:
http://store.modelpower.co.uk/lipo-safety-4-w.asp
http://www.bmfa.org/resources/lipobatteries.html
Information on battery recycling schemes:
http://www.wrap.org.uk/local_authorities/batteries/battery_recycling_information/index.html
Websites with information on many cell chemistries
http://www.powerstream.com/BatteryFAQ.html
http://www.mpoweruk.com/chemistries.htm
(Editors note: NiCd is the correct abbreviation for Nickel Cadmium. NiCad was in fact a trade name for Saft cells. Equally NiMH rather than NiMh is the correct abbreviation for Nickel Metal Hydride. Having said that, all these abbreviations have been used by advertisers from time to time as is the word Nicad as a generic term, so we have now decided to settle on NiCd and NiMH)




